Measuring the contribution of cell mechanics to the selective activation of fibroblasts by cancer
Fibroblasts are a heterogenous group of cells comprising of subpopulations that have been found to be activated in the stromal microenvironment that regulate tumor initiation and growth. The underlying mechanisms of such selective activation of fibroblasts are not understood. We hypothesized that the intrinsic geometric heterogeneity of fibroblasts modulates the nuclear mechanotransduction of signals from the microenvironment, resulting in their selective activation.
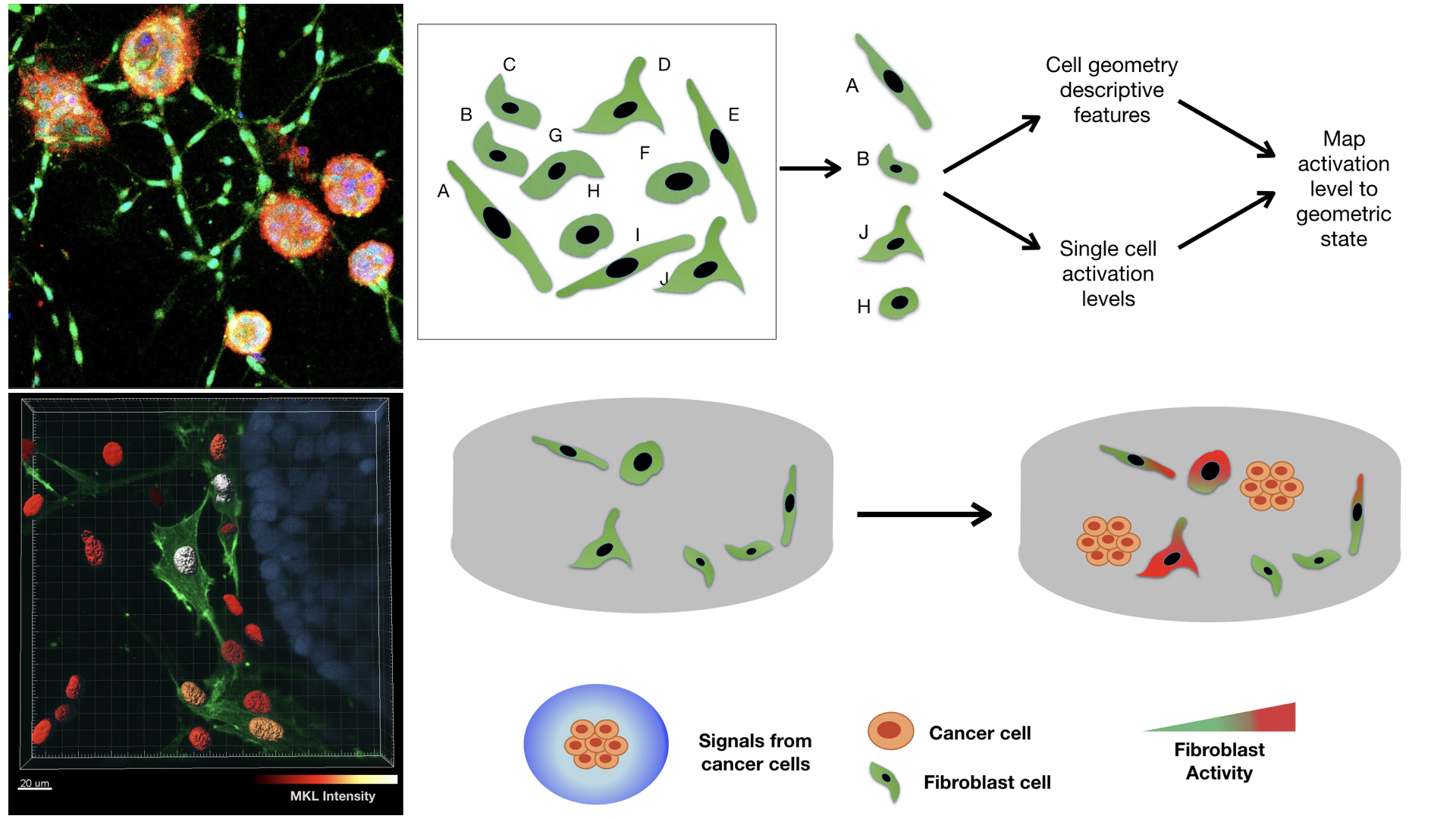
In order to understand the factors that contribute to the selective activation of stromal fibroblasts in the tumor niche, we developed 3D collagen tissue models of malignant breast cancer spheroids and fibroblasts to mimic the tumor stroma. We obtained high resolution images to quantify multiple nuclear morphological and chromatin organizational features. We used PCA-LDA to classify cells of different shapes with an accuracy of 95% and extracted the cell morphological index. The cell morphology index was then mapped to activation levels as measured by the nuclear abundance of transcription cofactor, MKL and protein levels of its target, αSMA for each cell.
Our results indicate the presence of activation-“primed” cell geometries that present higher activation levels which are further enhanced in the presence of stimuli from cancer cells. Further we show that by enriching the population of activation-primed cell geometric states by either increasing matrix rigidity or micro-patterning primed cell shapes, fibroblast activation levels can be increased.
Our results reveal important cellular geometric states that select for fibroblast activation within the heterogenous tumor microenvironment.Our study presents a framework for studying single cell heterogeneity and highlights the importance of the geometric state of fibroblasts in the interpretation of environmental signals.